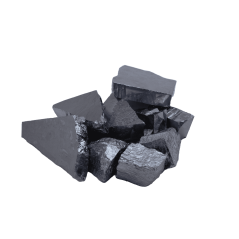
Silicium Granulat rein 99.9% Siliciumgranulat Si von 1gr bis 10kg
Silicium Granulat rein 99.9% Siliciumgranulat Si von 1gr bis 10kg
Silicon is a typical semi-metal from the carbon group. It has properties of metals and non-metals and is considered semiconductors. Pure silicon has very low electrical conductivity. On the other hand, its thermal conductivity is very high. Silicon is resistant to acids and water, but is soluble in hot alkali eyes under hydrogen development. Silicon has 23 isotopes. Three of these are stable and occur in nature. The single crystals of the silicon are cut into thin wafers, which are used for the production of microchips and semiconductors. Silicon is also used in most solar panels. As a semiconductor material, pure silicon is used in blue luminous diodes. Pure silicon serves as an alloy additive in aluminium alloys. Silicon as silicon carbide is a significant abrasive for the production of refractory materials.
Standard classifications:
- Name, symbol, atomic number — silicon, Si, 14.
- Assay — 99.9%.
Additional information:
- Density: 2,336 g/cm³.
- Melting point — 1410 °C.
- Mohs Hardness — 6.5
- Thermal conductivity — 148 W/(m · K).
- Electric. Conductivity: 2.52 – 10 cm S/m.
Pure silicon has the following features:
- Semi-metal with properties of metals and non-metals;
- Semiconductor;
- Very low electrical conductivity.
- Very high thermal conductivity.
- Acid and water resistance;
- soluble in hot alkaline eyes.